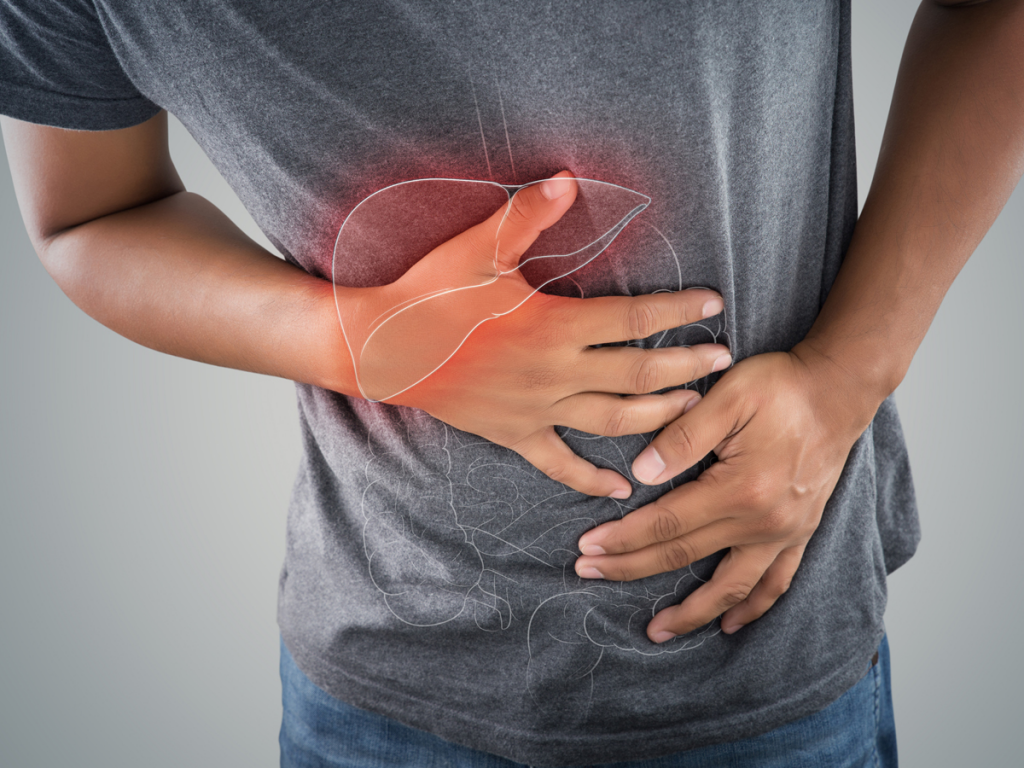
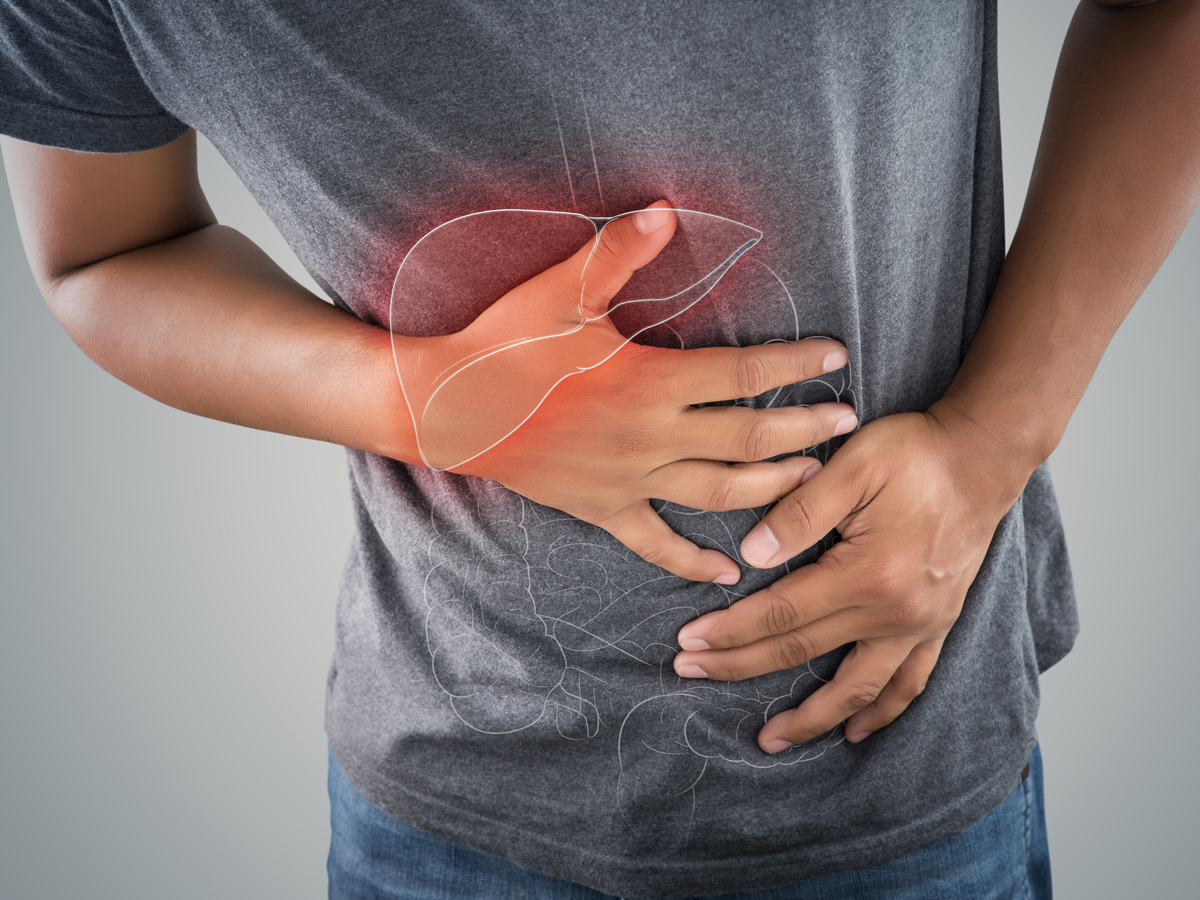
Acute pancreatitis is a leading cause of emergency department visits and gastrointestinal admissions in the U.S. It’s hard to diagnose and has no available treatment. For patients suffering from this condition, this means hospitalizations, ineffective medications, long term complications, and lost time with their families as well as work. Acute pancreatitis results in over 330,000 hospital admissions per year in the U.S., and approximately 5% of patients will die from the disease. Pancreatitis incidence is also on the rise, as it’s more common in obese patients with gallstones and related conditions that are increasing worldwide.
According to Dr. Gabi Hanna, a leading research physician, and CEO of Lamassu Pharma, the U.S. pharmaceutical industry is among the most technologically innovative sectors globally. However, the industry’s ability to offer breakthrough drugs and treatment protocols promptly is hampered by its own regulatory and commercial processes. The average time it takes a new treatment to get through clinical trials and achieve marketing approval is estimated to be over ten years by most industry experts. While proving the safety and efficacy of new drugs should be done with care, the need to accomplish this more quickly is also clear, particularly to patients waiting for life-saving treatments. The research process that will optimize the initial phases of new drug compound investigations is called translational research and is the main focus of Dr. Hanna’s work conducted through Lamassu Pharma.
Lamassu Pharma is breaking ground in translational research and has chosen to focus on acute pancreatitis with a boost from a Small Business Innovation Research (SBIR) grant provided by the National Institutes of Health (NIH). This funding is being directed straight toward developing its lead therapeutic compound, RABI-767, a novel small molecule lipase inhibitor licensed from the Mayo Foundation for Medical Education and Research, and is being developed with translational medicine techniques. The compound is being designed to fill a critical, unmet clinical need for a treatment for acute pancreatitis, mitigate the toxicity and organ failure associated with the disease that causes lengthy hospitalizations and death, saving both lives and resources.
Current research demonstrates that pancreatic microcirculation and capillary blood flow have long been suspected of playing a significant role in the course of acute pancreatitis. With this, manipulating the vasoconstrictor endothelin or its antagonist nitrogen monoxide could positively influence the outcome. However, these mechanisms take place very early in the disease course. Only prophylactic applications show an effect; this is useful in the setting of pancreas transplantation and endoscopic retrograde cholangiopancreatography, but not applicable for clinical use in acute pancreatitis therapy. Research then, focused on later pathophysiological stages of the disease, mainly on the process of adhesion and extravasation of leukocytes into the pancreatic tissue.
Dr. Hanna’s research is based on the knowledge of practicing physicians treating patients, who have recognized that acute pancreatitis leads to the release of pancreatic digestive enzymes. These enzymes wreak havoc in the body, and in severe cases, proteases and lipases are activated in a cascading effect, causing massive necrosis and organ failure. With no current treatment available to address these enzymes, Dr. Hanna and his team focus their clinical trial on the lipase inhibitor identified as a potential treatment by translational medicine researchers familiar with the science, the patient’s needs, and the disease. The first treatment vehicle of RABI-767 involves an injection directly into the pancreas. As this treatment protocol is studied further, adaptation into an IV or oral medication that is more convenient and marketable, will be explored. At this stage, the priority for Dr. Hanna and his team is getting the treatment directly to patients once the safety and efficacy data is analyzed.
The scientific approach of translational medicine, coupled with the business models of modern pharmaceutical and biotech companies, has the potential to bring new treatments to patients more quickly than ever before. By focusing on compounds with clear clinical benefits in practice, and conducting preclinical and definitive data-driven clinical studies, programs like this one have the potential to make translational medicine a reality. Lamassu Pharma’s work with RABI-767 is one example of the way translational medicine is being applied to real-world medical situations.

Founder Dinis Guarda
IntelligentHQ Your New Business Network.
IntelligentHQ is a Business network and an expert source for finance, capital markets and intelligence for thousands of global business professionals, startups, and companies.
We exist at the point of intersection between technology, social media, finance and innovation.
IntelligentHQ leverages innovation and scale of social digital technology, analytics, news, and distribution to create an unparalleled, full digital medium and social business networks spectrum.
IntelligentHQ is working hard, to become a trusted, and indispensable source of business news and analytics, within financial services and its associated supply chains and ecosystems