The World Health Organisation estimates 50 percent of drugs consumed in developing countries are counterfeit. Among them, the majority are anti-malarias and antibiotics. These fake drugs can harm the patients and fail to treat the diseases they are prescribed for. Moreover, using these drugs can create resistances to the original product.
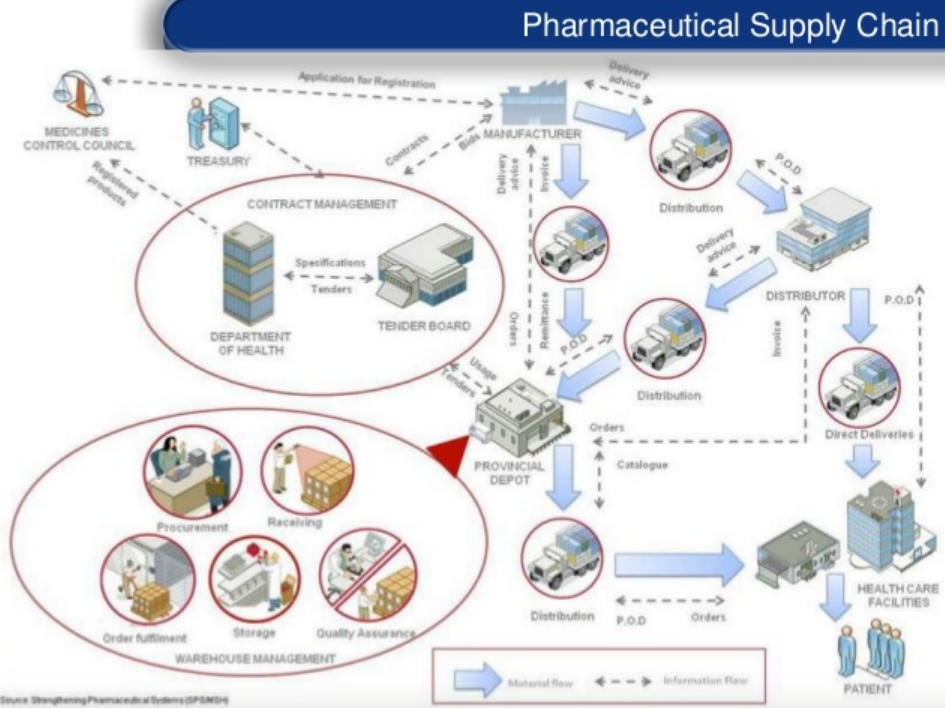
Although developed countries are less affected by this issue, the medical and pharmaceutical communities are implementing measures to provide secure drug traceability. One of the solutions could be product serialization, which consists in giving each product a unique identification number. For instance, in France, each medicine box has a Datamatrix with the batch number, the expiry date and a unique number called the CIP13. The objective is to allow each actor of the supply chain to verify that the drug they receive has the correct origin, composition and dosage. However, the great number of intermediaries makes it difficult for pharmaceutical companies, distributors or even final patients to have a complete vision over the supply chain. Here is an overview of the complex supply chain for pharmaceutical products:
In spite of the measures that are being taken, each year 700 000 deaths are caused by fake drug. Studies have shown that the pharmaceutical industry still struggles on two main points: interoperability between all the participants, from the manufacturer to the dispenser, and data management, to better integrate the serialization systems. Being able to avoid drug counterfeiting is just one of the reasons for which it is so critical to successfully track products down the supply chain. The great measures that are being adopted to insure that the right product arrives to the right patient at the right time also have several other objectives:
- Guarantee the quality of the product;
- Ensure an efficient tracking of the product at any time;
- Protect the patient and preserve public health;
- Make sure of the good compatibility between the patient and the drug;
- Be aware of the expiry date of the product;
- Allow a quick withdrawal of the batches in case of a problem.
A malfunction in the tracking system could have dramatic consequences on Public Health. The system nowadays is imperfect but several companies are trying to find a solution. Blockpharma is one of them. The French start-up is using blockchain technologies to come up with a new way of ensuring drug traceability.
IntelligentHQ met Fanny, co-founder of Blockpharma:
IntelligentHQ (IHQ): Can you tell me more about your company and your team?
Blockpharma (BP): We created our company, Crystalchain, last summer, after working on the project for over a year. The first case that we wanted to study was drug traceability and this is how we decided to launch Blockpharma. Our team is composed by successful entrepreneurs who had already launched several companies, engineers passionate about blockchain technologies and consultants that have a great knowledge of process in several industries and that help us analyse the opportunities and risks of blockchain technologies.
IHQ: Why did you decide to start with drug traceability?
BP: Traceability is one of the most obvious and direct applications of blockchain. Also, one of our associates worked in Africa and he gained a good knowledge of the problems created by fake drugs there. There is a strong need for solutions that fight this scourge and the people we met confirmed that it is a serious public health issue. Our solution, based on blockchain, brings security. There is no harmony in the processes used along the supply chain. Each actor knows what product they have and where they send it to but they don’t have any information on what happens to the drug afterwards. What we do is that we connect all this information together to tell the final consumer if his product followed a normal distribution channel.
IHQ: How does your solution function concretely?
BP: We develop an API that can be plugged into the information systems used by the pharmaceutical industry. Our API creates a bridge between the existing programs and the blockchain consortium. The laboratories release medicine boxes with QR codes that contain all the information about the product. The information, as well as each transaction, is stored on the blockchain. At the end of the supply chain, we can check the history of the product by simply using a smartphone.
IHQ: How do pharmaceutical companies react to you product?
BP: Drug traceability is a hot topic for the pharmaceutical industry. There is a new European directive that imposes to identify each medicine box in a unique way and to establish an effective tracking system. The companies are now trying to agree on a traceability protocol and find technical solutions. Fake drugs represent a considerable loss for them. Regarding the use of blockchain technologies, the response varies a lot. Some companies are quite reluctant to this solution because they associate it with Bitcoin. Only the Big Pharmas have internal teams that follow the trend but they don’t have any concrete applications yet.
IHQ: What stage in your development have you reached?
BP: Right now we are attacking the anti-malaria market, which is one of the most touched by drug counterfeiting in Africa. According to the Chirac Foundation, one in three anti-malaria drugs are fake. There are direct consequences on the patient: fake drugs create resistances to the real treatment. It is an important obstacle for therapeutic innovation and a sanitary emergency.
Our first prototype will be ready by the end of the year and we will launch a pilot in 2017 for a specific anti-malaria drug supply chain in an African country. We will bring together a pharmaceutical company with one or more wholesalers. For now, we all agreed on a first version that we want to launch and test quickly in order to see if blockchain is a good solution to this problem. After that, our goal is to add functions and improve the API according to the feedback that we will collect in the field.
IHQ: Have you identified any obstacles?
BP: The good thing is that there are no legal barriers for now. We don’t have enough hindsight to create any regulation; at this point it would just block us. Some companies try to develop standards to reassure the industry about the use of blockchain technologies. Today, we have very few initiatives; what we have are just proof-of-concept. However, the closer we will get to market-ready solutions, the more we will need regulation, especially in order to give a legal value to the information stored on the blockchain. On a more technical note, there are a few solutions that make it easier to develop applications using the blockchain. We chose the Ethereum blockchain because it is more adapted to companies. So, we are not concerned about the technical aspect, it is evolving fast. The main challenge is to convince the industry to adopt our product.
IHQ: What type of blockchain are you using?
BP: We chose a private blockchain. The blockpharma blockchain will only index trustworthy actors that we select. We don’t want everyone to be able to register drugs on our blockchain. That way, we can be sure that the drugs followed by Blockpharma are authentic. Depending on your position on the supply chain, you can have different access rights to the Blockpharma blockchain. Labs can register drugs whereas wholesalers can only approve transactions.
IHQ: Is blockchain a technology too recent to be successfully implemented?
From a technical point of view, blockchain is a young technology but it is growing very fast and we now have solutions easy to develop. However, what is still immature is the understanding others have of the technology and its applications. If you compare it with the Internet, at first it allowed people to gain access to information. But further down the road, it completely changed consumer habits. For blockchain, there are direct applications but understanding the transformations it will bring to society is still hard.
IHQ: Do you have any advice for future entrepreneurs?
BP: You have to hang on, believe as hard as you can in your idea, listen to the market and to customer feedback. The main point is that you confront the market as soon as you can, without trying to have a perfect product from the beginning. We have a product that will compete with solutions built by big companies and that have been used for years. But we clearly show why our solution is better than theirs and it convinces people. You have to go fast, proceed in short cycles and test your products fast.
IHQ: How do you feel about the French start-up ecosystem?
BP: Regarding blockchain, we need initiatives in France. France missed the Internet revolution but it cannot miss this one. Industries and companies have to be more aware of this technology. Of course, the French ecosystem is smaller than the American one but people are interested in this topic, the government is talking about it and they wish to encourage all the initiatives by not putting up any regulation. The BPI (Public Investment Bank) actually launched a competition to develop applications connected to the blockchain.
Many actors from the medical and pharmaceutical communities believe that blockchain is still at a very early stage and it cannot be used in the healthcare system yet. In fact, there are two categories of people: those who believe unconditionally in the fact that blockchain will change healthcare and those who are more sceptical. The reality is slightly more nuanced: everything depends on the application for which blockchain is used. Let’s take two examples: electronic medical records and drug traceability. On one hand, it is obvious that trying to modify the way medical information is stored and used is a complicated task. Some doctors are hardly starting to use digital technologies to keep their patient’s files.
How could one even talk to them about blockchain? Moreover, governments are heavily investing in electronic medical records and for now, they are not opened to a new solution that would question everything they have done so far. Indeed, the usage of blockchain technologies for keeping medical records would need to rethink the whole way the current system works. “A lot of the time, these entrepreneurs talk about things that aren’t actually doable. I don’t think they understand how busy our day is and how the system underneath actually works.” explains Dr Alyssa Hoverson Schott, a physician at Sanford Health. On the other hand, if you target specific and precise areas of application, you can find cases in which it is very realistic to use blockchain. A good example for this is drug traceability. There is a real need and no effective solution for now. And it is not just a supposition. Companies in this area like Blockpharma or Block Verify (UK-based blockchain start-up for drug traceability) are launching prototypes.
One cannot generalize and assume that because blockchain is a recent technology it cannot be used in the immutable healthcare system. If no one dares to try, it is clear that nothing will ever change. But some start-us are rising to the challenge and, by attacking niche markets, they get a step closer to initiating the transformation our healthcare system so desperately needs.

My name is Anca Petre and I am a Pharm. D and Business double degree candidate at Université Paris-Saclay and INSEEC Business School in London and Paris. In my own work, I primarily focus on the crossroads between healthcare and digital technologies. I am currently working on a research about the possible applications of Blockchain in Healthcare and I contribute to Blockchain Age and Intelligent HQ.
I particularly enjoy working the sectors’s startup environment. I have previously worked at e-ssencials, a startup which develops digital solutions for patients with chronic diseases. I have also participated in three hackathons to help develop and further innovate the healthcare circuit.
I worked at Sanofi in the Biotechnologies Department where I successfully managed to reduce the production time by half for several products.
At university, I am the founder and president of the Speak Up ! Association which allows over 500 students to improve their public speaking skills.











